Cdc Orders Mass Retraction
In a recent development that has sent shockwaves through the medical community and sparked widespread debate, the Centers for Disease Control and Prevention (CDC) has issued an unprecedented order for a mass retraction of scientific findings and recommendations. This bold move, which affects a significant number of studies and guidelines, has raised crucial questions about the integrity and reliability of scientific research and its impact on public health.
The CDC's decision to retract a substantial body of work has highlighted the importance of scientific integrity and the need for rigorous review processes. While retraction is not an uncommon practice in the scientific realm, the scale and scope of this particular retraction are exceptional, making it a highly significant event in the history of medical research.
The implications of this retraction are far-reaching and will likely have a profound impact on various aspects of public health and medical practice. It underscores the complex and evolving nature of scientific understanding and the challenges inherent in translating research into actionable recommendations.
Understanding the CDC's Decision: A Comprehensive Overview
The CDC's decision to order a mass retraction was not taken lightly and was the result of an extensive review process. The agency has long been regarded as a cornerstone of public health, providing critical guidance and recommendations based on rigorous scientific research. However, recent events have cast a spotlight on the need for heightened scrutiny and transparency in scientific research.
The retraction process began with an internal review initiated by the CDC itself, which identified a range of concerns and issues with certain studies and guidelines. These concerns ranged from methodological flaws and data inconsistencies to potential conflicts of interest and a lack of transparency in reporting. The review process was meticulous, involving expert panels and external reviewers to ensure an unbiased and comprehensive assessment.
As a result of this rigorous review, the CDC concluded that a significant number of studies and guidelines did not meet the agency's rigorous standards for scientific integrity. This decision was not taken lightly, as it acknowledges the potential impact on public health and the trust placed in the CDC's recommendations. The agency's commitment to scientific integrity and public health drove this difficult but necessary decision.
The CDC's retraction encompasses a wide range of studies and guidelines, including those related to infectious diseases, chronic conditions, and public health interventions. While the full list of retracted studies and guidelines is not yet publicly available, preliminary reports indicate that the retraction covers a substantial body of work published over several years.
One notable example of a retracted study is a research paper that linked a specific environmental factor to an increased risk of a particular disease. This study, which received significant media attention and influenced public health policies, has now been retracted due to concerns about the validity of its findings. The CDC's review revealed methodological flaws and a lack of robust evidence to support the study's conclusions.
Similarly, certain guidelines related to preventive healthcare measures, such as vaccination schedules and screening protocols, have also been retracted. These guidelines, which were widely adopted by healthcare providers and public health officials, are now under scrutiny due to concerns about their scientific basis and potential impact on patient care.
The Impact on Public Health and Medical Practice
The CDC's mass retraction has significant implications for public health and medical practice. It underscores the importance of scientific integrity and the need for healthcare professionals and policymakers to critically evaluate the evidence base for any recommendation or guideline.
For healthcare providers, the retraction serves as a reminder of the importance of staying abreast of the latest scientific developments and critically appraising the evidence behind clinical guidelines. It emphasizes the need for a nuanced understanding of the strengths and limitations of scientific research and its translation into clinical practice.
Public health officials and policymakers must also carefully reconsider the impact of the retracted studies and guidelines on their existing strategies and policies. While the CDC's retraction may lead to adjustments in certain public health initiatives, it also presents an opportunity to strengthen the scientific foundation of public health practices and ensure that they are based on robust and reliable evidence.
Furthermore, the retraction highlights the need for improved communication and collaboration between scientists, healthcare professionals, and the public. Clear and transparent communication about the reasons for retraction and the implications for public health is essential to maintain trust and ensure that the public understands the evolving nature of scientific knowledge.
The Role of Scientific Integrity and Peer Review
The CDC's decision to retract a mass of studies and guidelines underscores the critical importance of scientific integrity and the role of peer review in ensuring the reliability of scientific research.
Scientific integrity refers to the adherence to ethical principles and rigorous scientific methods in the conduct and reporting of research. It encompasses transparency, accuracy, and the avoidance of bias or conflicts of interest. Peer review, a fundamental process in scientific publishing, involves the critical evaluation of research by independent experts in the field. This process is designed to ensure that published research meets high standards of quality and integrity.
The CDC's internal review process, which led to the mass retraction, serves as a testament to the agency's commitment to scientific integrity. By identifying and addressing concerns with certain studies and guidelines, the CDC is upholding its responsibility to ensure that its recommendations are based on the best available evidence and are in the public's best interest.
However, the mass retraction also raises important questions about the effectiveness of the peer review process and the need for continuous improvement. While peer review is a vital mechanism for maintaining scientific integrity, it is not infallible. The retraction of a substantial body of work highlights the potential limitations of the current peer review system and the need for ongoing evaluation and refinement.
Moving forward, the scientific community and research institutions must continue to prioritize scientific integrity and strive for continuous improvement in the peer review process. This includes fostering a culture of transparency and accountability, promoting rigorous scientific methods, and encouraging open dialogue and debate within the scientific community.
The Future of Scientific Research and Public Health
The CDC's mass retraction serves as a pivotal moment in the history of scientific research and public health. It underscores the complex and evolving nature of scientific understanding and the ongoing need for rigorous scrutiny and continuous improvement.
As the scientific community reflects on this significant event, several key takeaways emerge. First and foremost, scientific integrity must remain a paramount concern. Researchers, institutions, and funding bodies must prioritize ethical conduct and rigorous methods to ensure the reliability and trustworthiness of scientific research.
Secondly, the peer review process, while essential, should be continually evaluated and refined. The scientific community should explore innovative approaches to peer review, such as open peer review and post-publication review, to enhance transparency and accountability.
Additionally, the retraction highlights the importance of interdisciplinary collaboration and the integration of diverse perspectives in scientific research. By fostering collaboration across disciplines, researchers can address complex public health challenges more effectively and ensure that their work is grounded in a comprehensive understanding of the issues at hand.
Finally, the CDC's retraction emphasizes the need for ongoing education and training in scientific integrity and research methods. Researchers, particularly early-career investigators, should receive comprehensive training in these areas to ensure that they are equipped with the skills and knowledge necessary to conduct high-quality research and contribute to the advancement of scientific knowledge.
As the scientific community navigates the aftermath of the CDC's mass retraction, it is crucial to maintain a balanced perspective. While the retraction highlights areas for improvement, it also underscores the self-correcting nature of science and the commitment of the scientific community to upholding the highest standards of integrity and excellence.
Conclusion
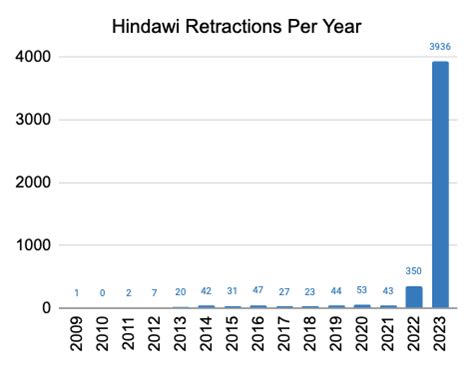
The CDC's order for a mass retraction of scientific findings and recommendations is a watershed moment in the history of medical research. It underscores the complex interplay between scientific integrity, public health, and the evolving nature of scientific understanding. While the implications of this retraction are far-reaching, they also present an opportunity for the scientific community to strengthen its commitment to integrity, improve peer review processes, and foster interdisciplinary collaboration.
As we move forward, it is essential to maintain a critical yet constructive dialogue about the role of science in public health and the ongoing efforts to ensure that scientific research is conducted and communicated with the utmost integrity and transparency. The CDC's retraction serves as a powerful reminder of the importance of these principles and the ongoing work required to uphold them.
What is the primary reason for the CDC’s decision to order a mass retraction of scientific findings and recommendations?
+The primary reason for the CDC’s decision is to uphold scientific integrity and ensure that its recommendations are based on robust and reliable evidence. The agency’s rigorous review process identified concerns with certain studies and guidelines, including methodological flaws, data inconsistencies, and potential conflicts of interest.
How will the mass retraction impact public health and medical practice?
+The retraction will have significant implications for public health and medical practice. It underscores the importance of critically evaluating the evidence base for any recommendation or guideline. Healthcare providers and policymakers will need to reconsider the impact of the retracted studies and guidelines on their existing strategies and policies.
What does the CDC’s retraction say about the state of scientific integrity and peer review processes?
+The CDC’s retraction highlights the critical importance of scientific integrity and the role of peer review in ensuring the reliability of scientific research. While the retraction underscores the need for continuous improvement in the peer review process, it also serves as a testament to the agency’s commitment to scientific integrity and public health.
How can the scientific community move forward in light of the CDC’s mass retraction?
+The scientific community can move forward by prioritizing scientific integrity, continuously improving the peer review process, fostering interdisciplinary collaboration, and providing comprehensive education and training in scientific integrity and research methods. This will help ensure that scientific research remains rigorous, reliable, and grounded in ethical principles.



